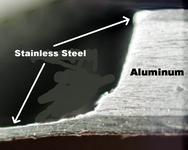
We recently came across this post with a neat trick on removing hard water stains from stainless steel.
A coating of mineral deposits from hard water is a fact of life in stainless steel cookware that is used to cook with water that has any hardness. With our sauce pans, we often have to scrub the hard water coating off about every other time we boil water for soft-boiled eggs. Our water is around 40+ grains of hardness, and we have a water softener. Still, the hard water coating is a fact of life. Here is what it looks like:

Usually, we just use a green Scotch-Brite pad to scrub it out. It does dull the inside, but if you cook with metal utensils, or have had your sauce pan for any appreciable time, the inside is already fairly dull.
This new trick involves using vinegar (they say apple cider vinegar but I’m sure white vinegar or any other type would do well) and a scrunched up piece of aluminum. We tried it with white vinegar. The first thing to notice is that as you scrub, a black residue is left on the bottom of the pan.

That makes me think there is some kind of chemical reaction happening that involves the hard water residue (calcium or magnesium carbonate), the vinegar (acetic acid) and aluminum (just Al). It is known that acetic acid will dissolve calcium carbonate (and presumably other mineral carbonates), albeit, in my experience, slowly. It is also my experience that scrubbing hard water stains with a soft scrubbing material (like a non-metal kitchen scrubbing sponge) will hasten the dissolving of the hard water stains (abrasion and agitation). Is the aluminum just acting as an abrasive surface, or is it doing more to chemically remove the hard water?
In any event, the trick seems to work quite well.

Given the softness of aluminum, I can only imagine that it is less abrasive a method than the Scotch-Brite scrubbing.
Speaking of aluminum, here is an interesting side fact; if you’ve ever wondered why one side of aluminum foil is shiny while the other side is dull, the short explanation is that, aluminum goes through rollers to make it progressively thinner and thinner, while stretching it out, to get it from a thick ingot, to being thin enough for aluminum foil. On the last step, two sheets of foil go through the same set of rollers. Where each sheet comes in contact with the roller becomes the shiny side (it is being pressed on by a very hard steel surface) while the side where the two pieces of aluminum touch each other becomes the duller side. Here is a longer explanation.
You might also ask if the shiny side or the dull side is better for keeping in the heat when you cover a dish with aluminum foil. The answer is, it doesn’t matter. My daughter just did a science experiment where she created a device to measure the reflectivity of various materials, one of them being aluminum foil. The shiny and dull sides had virtually identical reflectivity, and reflecting the infra-red energy back into the dish that is covered is what helps keep it warm.